

| Item | Index |
|---|---|
| Appearance | Colorless transparent viscous liquid |
| Content(%) | 97.0—102% |
| Caprolactam(%) | ≤0.1 |
| Bromide(%) | ≤0.1 |
| Heavy metal(ppm) | ≤10 |
| Refractivity | 1.470-1.473 |
| Relative density | 0.906-0.926 |
| PH value | Neutral |
| Packing | in 10kg carton(500ml/bottle), 20kg paper barrel, 25kg drum |
|---|---|
| Storage | 20℃, 2 years. |
| Shipping | Room temperature in China; may vary elsewhere |
Tel: 0086-25-52397805
E-mail: info@liwei-chem.com


| Common Names | Laurocapram | Azone | 1-dodecyl-hexahydro-azepin-2-one | ||
|---|---|---|---|
| Structure |
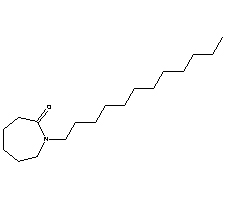
|
||
| CAS No. | 59227-89-3 | Boiling Point (℃) | 404.9±14.0 °C |
| Molecular Weight | 281.477 | Melting Point (℃) | '-7ºC |
| Appearance | Colorless liquid | Vapor Specific Gravity | 0.9±0.1 g/cm3 |
| HS Code | 2933990099 | Flash Point (℃) | 165.2±10.7 °C |
| Solubility | Insoluble in water, soluble in ethanol, ether, acetone and benzene | Autoignition Temperature (℃) | |
| Safety Phrases | |||
|---|---|---|---|
| RIDADR | |||
| WGK Germany | |||
| Packaging Group | |||
| Hazard Class | |||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention. |
1) In a reaction vessel equipped with a stirrer, add dodecane bromide and solvent oil, control the stirring speed to 30120rpm, heat the temperature of the solvent oil solution to 4595°C, add caprolactam, tetrabutylammonium bromide TBAB, hydroxide Sodium, reaction 38 hours; Bromododecane: caprolactam: sodium hydroxide is 1mol: 0.81.4mol: 0.82mol, solvent oil is greater than the quality of total raw material of reaction, a small amount of phase transfer catalyst TBAB tetrabutyl ammonium bromide;
2) Add an appropriate amount of water to the reaction solution to dissolve sodium hydroxide and the sodium bromide generated by the reaction, let stand to separate layers, and separate the lower aqueous solution; after the upper organic layer recovers the solvent oil under normal pressure, it is distilled under reduced pressure to obtain the finished product of azone. More than 92%, Azone content is more than 95%.
Penetration Enhancer
Laurocapram, known as N-laurylcaprolactam, acridone, and azone, is chemically named 1-dodecylazacyclohept-2-one. Laurocapram skin penetration enhancer is a new type of efficient and safe penetration enhancer. It is a colorless, odorless, and transparent liquid at room temperature, slightly viscous and odorless. It forms an emulsion with water, is easily soluble in various organic solvents, and has good lubricity. It can promote the activity of hydrophilic and lipophilic drug products or the penetration of nutrients in cosmetics into the skin, greatly reducing the retention time of drugs in the stratum corneum, significantly enhancing the efficacy of drugs and the effectiveness of cosmetics, thereby reducing the dosage of main drugs and reducing costs. There is a lag phenomenon in the promotion of transdermal absorption of laurocapram. Laurocapram has significant transdermal and permeation-enhancing effects on both hydrophilic and lipophilic drugs, and has good permeation-enhancing effects in both emulsion and colloidal states. It is particularly effective on various plant extracts and alkaloids. The transdermal permeation-promoting effect is stronger than that of dmso dimethyl sulfoxide, and the permeation-promoting effect on 8-bromocyclic nucleotides and 1% laurocapram is 12 times that of 50% dimethyl sulfoxide. Commonly used in combination with propylene glycol to produce synergistic effects, the optimal transdermal concentration is 0.1% to 5%, safe, low toxicity, and nonirritating. Stable chemical properties, stored in the dark at room temperature for over four years. This product can be considered as a complex of decyl methyl sulfoxide and pyrrolidone and is a good new type of transdermal enhancer with low skin irritation. It can promote the transdermal absorption of many drugs such as corticosteroids, indomethacin, fluorouracil, hydroquinone, etc., allowing the drug to pass through the skin barrier, increase local and systemic blood drug concentration, and improve the bioavailability of the preparation. It has transdermal effects on both lipophilic and hydrophilic drugs. And it can soften the keratin and enhance its permeability. It still has anti-inflammatory, analgesic, and antipruritic effects. Laurocapram has low toxicity and oral LD50>7g/kg. It should not be combined with strong acids or vaseline to avoid reducing the therapeutic effect.